In 2011, a horticulturist named Mathias Maier stumbled across an unusual mutant of a Venus flytrap, a carnivorous plant that traps and feeds on insects. Scientists recently discovered that the typical Venus flytrap can actually "count" to five, sparking further research on how the plant manages this remarkable feat. The mutant flytrap might hold the key. According to a new paper published in the journal Current Biology, this mutant flytrap doesn't snap closed in response to stimulation like typical Venus flytraps.
"This mutant has obviously forgotten how to count, which is why I named it Dyscalculia (DYSC)," said co-author Rainer Hedrich, a biophysicist at Julius-Maximilians-Universität Würzburg (JMU) in Bavaria, Germany. (It had previously been called "ERROR.")
As we've reported previously, the Venus flytrap attracts its prey with a pleasing fruity scent. When an insect lands on a leaf, it stimulates the highly sensitive trigger hairs that line the leaf. When the pressure becomes strong enough to bend those hairs, the plant will snap its leaves shut and trap the insect inside. Long cilia grab and hold the insect in place, much like fingers, as the plant begins to secrete digestive juices. The insect is digested slowly over five to 12 days, after which the trap reopens, releasing the dried-out husk of the insect into the wind.
In 2016, Hedrich led the team of German scientists who discovered that the Venus flytrap could actually "count" the number of times something touches its hair-lined leaves—an ability that helps the plant distinguish between the presence of prey and a small nut or stone, or even a dead insect. The scientists zapped the leaves of test plants with mechano-electric pulses of different intensities and measured the responses. It turns out that the plant detects that first "action potential" but doesn't snap shut right away, waiting until a second zap confirms the presence of actual prey, at which point the trap closes.
But the Venus flytrap doesn't close all the way and produces digestive enzymes to consume the prey until the hairs are triggered three more times (for a total of five stimuli). The German scientists likened this behavior to performing a rudimentary cost-to-benefit analysis, in which the triggering stimuli help the Venus flytrap determine the size and nutritional content of any potential prey struggling in its maw and whether it's worth the effort. If not, the trap will release whatever has been caught within 12 hours or so.
In 2020, Japanese scientists genetically altered a Venus flytrap so that it glows green in response to outside stimulation, yielding important clues about how the plant's short-term "memory" works. They introduced a gene for a calcium sensor protein called GCaMP6, which glows green whenever it binds to calcium. That green fluorescence allowed the team to visually track the changes in calcium concentrations in response to stimulating the plant's sensitive hairs with a needle.
The results supported the hypothesis that the first stimulus triggers the release of calcium, but the concentration doesn't reach the critical threshold that signals the trap to close without a second influx of calcium from a second stimulus. That second stimulus has to occur within 30 seconds, however, since the calcium concentrations decrease over time. If it takes longer than 30 seconds between the first and second stimuli, the trap won't close. So the waxing and waning of calcium concentrations in the leaf cells really do seem to serve as a kind of short-term memory for the Venus flytrap, though precisely how calcium concentrations work with the plant's electrical network remains unclear.
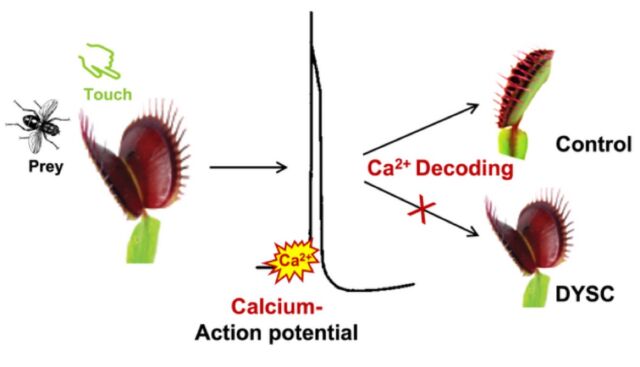
That doesn't seem to be the case with DYSC, even though it is otherwise "essentially indistinguishable" from Venus flytraps in the wild. DYSC does not close in response to two sensory stimuli, nor does it process its prey in response to additional stimuli. Naturally, Hedrich et al. wanted to find out why. They purchased wild Venus flytraps and the mutant DYSC flytraps and performed parallel experiments: both mechanically stimulating the plants and measuring the action potentials, and spraying the plants with a contact hormone called jasmonic acid, which is crucial for the processing of prey.
Hedrich and his team found that the mutation did not seem to affect either the action potential or the underlying calcium signal in the first two-count stage of the process. The action potentials fire, yet the trap doesn't snap shut, suggesting that the touch-activation of calcium signaling is being suppressed. Furthermore, the scientists suspected a defect that affected the decoding of the calcium signal. Administering jasmonic acid didn't fix the problem with the failure of the rapid trap closure, but it did restore the ability to process prey.
Co-author Ines Kreuzer next examined gene expression patterns in the mutant genes to spot any changes that might account for this. She was able to narrow the likely suspects down to a few decoding components, which bind to calcium and subsequently modify certain effector proteins—most notably an enzyme called LOX3, which plays a vital role in the biosynthesis of jasmonic acid. The next step is to look more closely at the modified proteins and change their activity when prey comes into contact with DYSC. "In this way, we want to close the circle and find out what the plant does to distinguish numbers from each other—i.e., how it counts," said Hedrich.
DOI: Current Biology, 2023. 10.1016/j.cub.2022.12.058 (About DOIs).
Listing image by Naturfoto Honal | Getty
reader comments
47